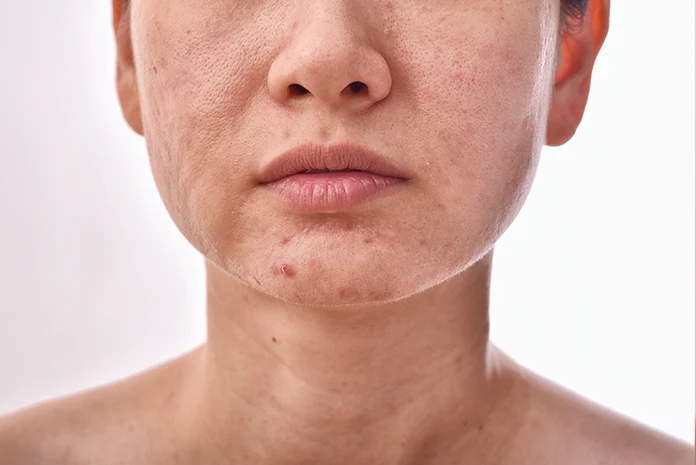
Who Would Qualify First for an Acne Vaccine
Acne affects millions of people around the world, causing painful pimples, red bumps, and scars that can last a lifetime. Right now, there is no approved acne vaccine, but early research and trials for new treatments give clues about who might qualify first if one gets developed. Scientists focus on those with the worst cases because they have the most to gain from a breakthrough.
Imagine a vaccine that stops acne at its root by targeting the bacteria or inflammation that cause it. Current studies show that severe or stubborn acne gets priority in testing new options. For example, papulopustular acne, which means red inflamed bumps and pus-filled pimples, is a main target in ongoing trials. These trials test gels with ingredients like clindamycin, an antibiotic that fights bacteria, and tretinoin, a vitamin A form that speeds up skin renewal. Participants in these studies have moderate to severe lesions that do not go away with basic creams or washes.[1]
Experts also point to people with acne vulgaris that resists other treatments. This includes cases where pimples lead to scarring, hurt mental health, or keep coming back despite months of therapy. A big study using expert opinions is creating rules for isotretinoin, a strong oral medicine for tough acne. It highlights who needs it most: those with severe breakouts, high scarring risk, or big emotional impact from their skin. If a vaccine works like this medicine, the same group would likely line up first for shots.[2]
Pharma companies like Pfizer are already running early tests on acne treatments. Their recent trial checked safety and first signs of improvement in people with active acne. These phase one studies often start with adults who have clear symptoms but no other major health issues. A vaccine trial would follow suit, picking healthy volunteers aged 12 to 40, the peak years for acne, especially teens and young adults whose hormones trigger outbreaks.[3]
Why not everyone? Trials need to prove a vaccine is safe before wider use. First in line would be those at highest risk who have failed standard care, like prescription topicals or pills. Kids under 12 or pregnant people might wait longer due to safety worries. Dermatology reviews from 2025 note big pushes in trials, but vaccines lag behind gels and pills because acne is not always seen as an infectious disease needing shots.[5]
In short, if an acne vaccine nears approval, expect severe cases to qualify first. Teens with scarring risks or adults with treatment-resistant pimples would top the list, based on how trials pick people today.
Sources
https://clinicaltrials.eu/trial/study-on-the-effectiveness-and-safety-of-clindamycin-and-tretinoin-gel-for-patients-with-papulopustular-acne/
https://clinicaltrials.gov/study/NCT07296523
https://www.tipranks.com/news/company-announcements/pfizer-completes-early-stage-acne-trial-signaling-quiet-expansion-in-dermatology
https://clinicaltrials.gov/study/NCT07296536
https://www.hcplive.com/view/dermatology-in-2025-year-in-review